Despite the introduction of an effective treatment 40 years ago, tuberculosis (TB) is spreading globally. Over the past 10 years, recent efforts from various groups have generated a promising TB drug pipeline (Poce et al. Eur.J.Med.Chem.2014, 86, 335-351). SQ109 (N-geranyl-N-(2-adamantyl)ethane-1,2-diamine) proved to be more active than ethambutol both in vitro and in vivo and has more favorable pharmacokinetic properties and, importantly, has been shown to accumulate in the lung, the site of M. tuberculosis infection. SQ109 inhibits Mmpl3 lipid transporter. It proved to be active against both drug-susceptible (MIC ranging from 0.2 to 0.39 mg/mL) and drug-resistance (MIC ranging from 0.2 to 0.78 mg/mL) M. tuberculosis strains in vitro and clinical isolates of M. tuberculosis. SQ109 showed a distinctive pharmacological profile in mice, while it showed poor oral bioavailability in rats and dogs. In both single and multi-dose Phase I and Phase II clinical trials, SQ109 proved to be both safe and well- tolerated (Zumla at al. Nat. Rev.2013, 12, 388-404; Sacksteder et al. Future Microbiol.2012, 7, 823-837; Jia et al. Br. J. Pharmacol.2006, 147, 476-485).
Recently an x-ray structure of SQ109 in complex with Mmpl3 provided a platform for structure-based drug design (Zhang, B. et al. Cell. 2019, 176, 636-648) (Figure 1).
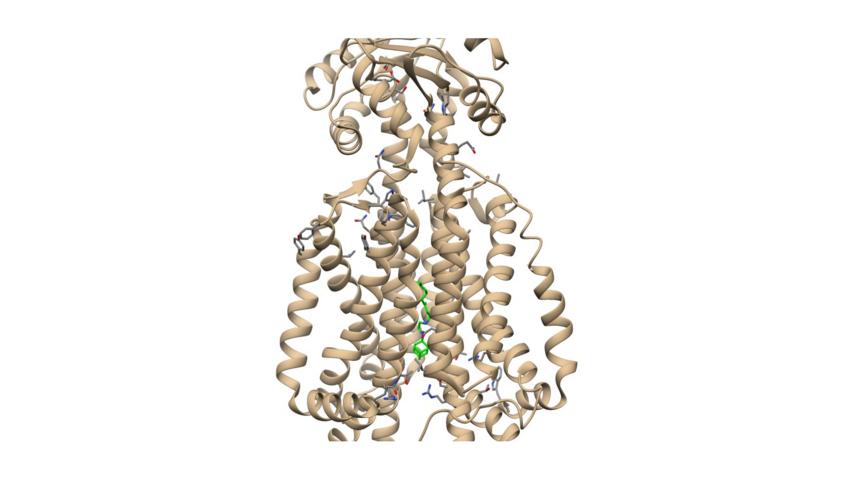
Figure 1. SQ109 complex with Mmpl3 protein (ligand's carbon atoms depicted in green).
Using the MmpL3-SQ109 structure we designed and synthesized new analogues as a part of an ongoing project to develop highly potent anti-tuberculosis therapeutics, with SQ109 being optimized (Scheme 1).
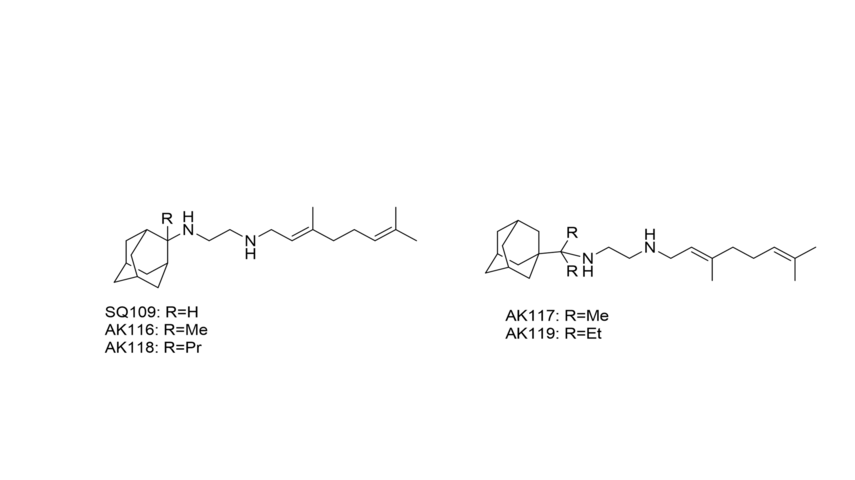
Scheme 1. Design of new SQ109 derivatives targeting the Mmpl3 transporter.
To achieve these analogues of SQ109, geranylamine was synthesized from the low cost geraniol. The desired aminoadamantane conjugates were obtained through a mild reduction, applied for the first time in these derivatives. The derivatives were against TB (in collaboration with Prof. E. Oldfield, USA) and M. smegmatis. Also against trypanosome brucei (in collaboration with Dr J. Kelly, London). The currently obtained results showed that the derivatives are equipotent compared to SQ109 against TB and M. smegmatis and 10-fold more potent against T. brucei. We also have started a collaboration on the determination of the crystal structures of AK116-AK118. Since SQ109 are known to perturb membrane potential which is important for ATP levels inside the cytoplasm we investigated the interaction of these molecules with model membranes using DSC and observed that AK116-AK118 and SQ109 effect a significant pertubation to DMPC membranes.